Pharmaceutical Industry Applications for Dynamic Vapor Sorption
In the pharmaceutical industry, materials come into contact with water in many ways, such as during processing steps like crystallization, spray drying, and wet granulation. Additionally, pharmaceutical raw materials or finished products are usually exposed to a humid atmosphere during storage and transport, which leads to interactions between solid components and water. In multi-component formulations, water can migrate between moist and dry components. Regarding the compaction behavior during tableting, a certain moisture content is required to produce satisfying products. In general, however, the exposure to moisture causes undesirable product changes. Therefore, it is of utmost importance for the development of new drugs to have comprehensive and detailed information on water-solid interactions and the influence of moisture on the physicochemical properties of Active Pharmaceutical Ingredients (APIs) and excipients.
Hydrate Formation
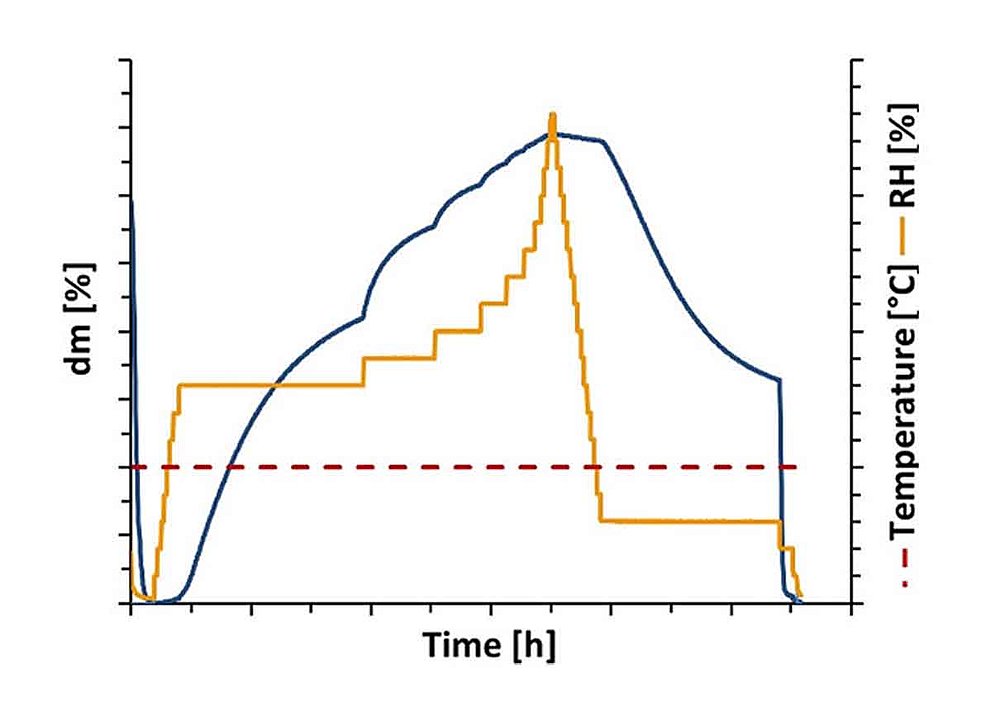
Many crystalline solids form hydrates in the presence of water. The water molecules can be incorporated into the crystal lattice in stoichiometric or non-stoichiometric proportions. Typically, a hydrate is only stable within a certain temperature and relative humidity range. Upon dehydration, a hydrate may reversibly transform into an anhydrous or a less hydrated form. The hydrate form affects the physical and chemical properties of a crystalline solid such as solubility, stability and processing properties. Therefore, the hydrate form of an Active Pharmaceutical Ingredient (API) or excipient plays a significant role in the development of new drugs in connection with the bioavailability and the manufacturing process of the drug. In this context, dynamic water vapor sorption analysis enables the precise characterization of different hydrate states and their stability as a function of temperature and relative humidity. This allows new formulations, manufacturing processes and storage conditions to be adapted to specific requirements.
Amorphous Content
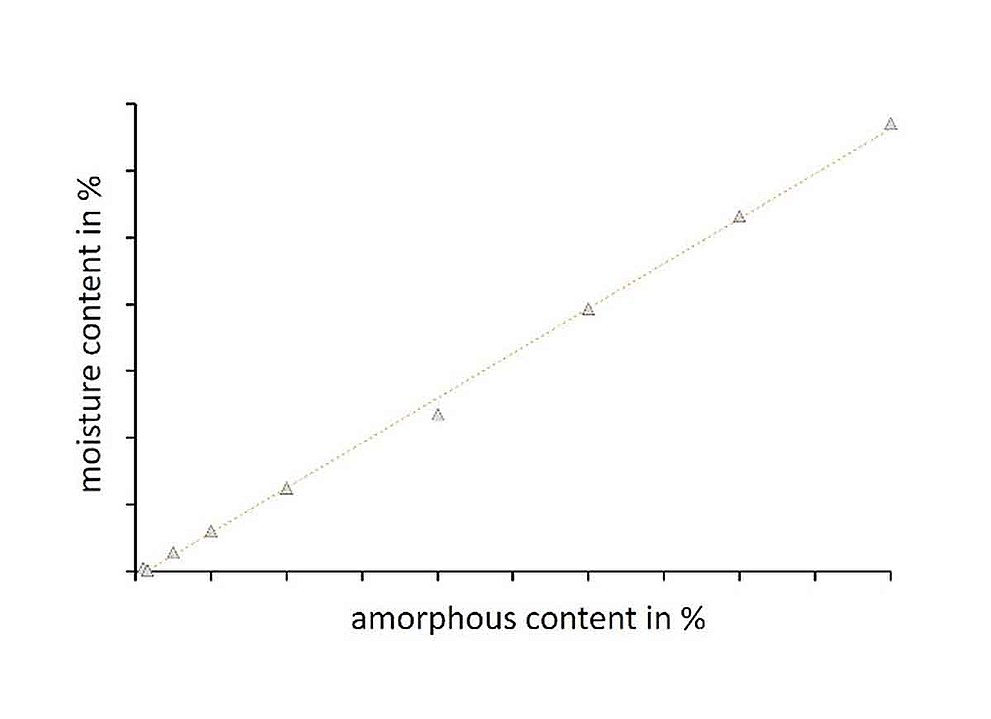
The quantification of the amorphous fraction is important in pharmaceutical and food research, as even the presence of small amorphous fractions can result in a significant change in product behavior. Since the amorphous state of a product has a higher hygroscopicity and thus water absorption compared to the crystalline state, dynamic water vapor sorption is ideally suited to quantify and to characterize the product-specific recrystallization kinetics as a function of temperature and humidity. The application notes provided below use practical examples to demonstrate both the analysis and the evaluation of DVS measurements to determine amorphous fractions.


