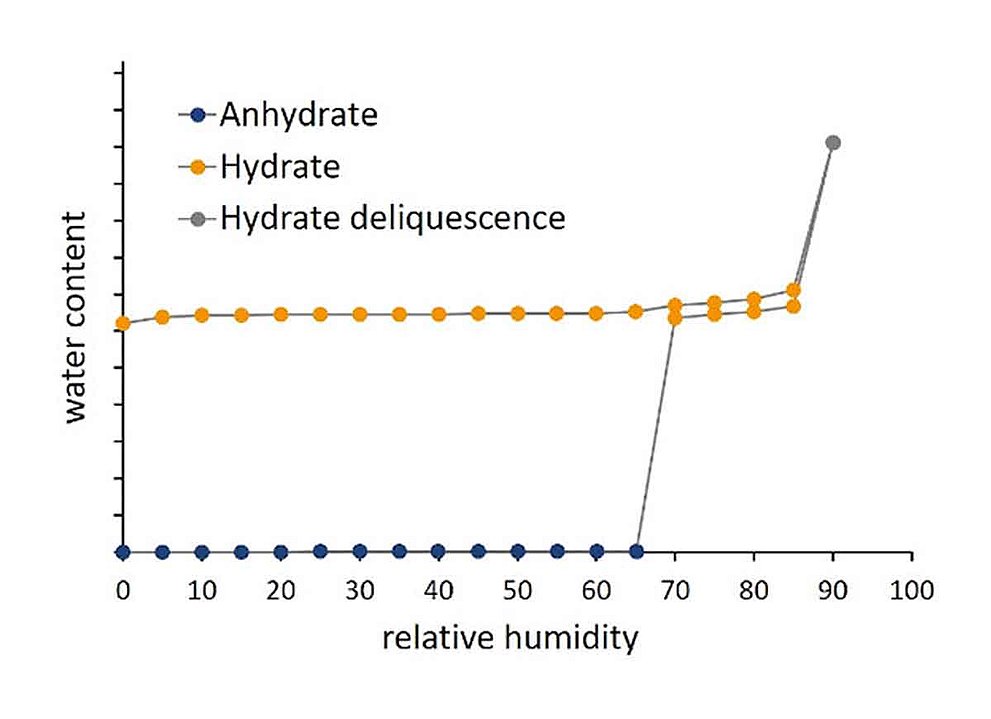
Hydrate Formation
Many crystalline solids form hydrates in the presence of water. The water molecules can be incorporated into the crystal lattice in stoichiometric or non-stoichiometric proportions. Typically, a hydrate is only stable within a certain temperature and relative humidity range. Upon dehydration, a hydrate may reversibly transform into an anhydrous or a less hydrated form. The hydrate form affects the physical and chemical properties of a crystalline solid such as solubility, stability and processing properties. Therefore, the hydrate form of an Active Pharmaceutical Ingredient (API) or excipient plays a significant role in the development of new drugs in connection with the bioavailability and the manufacturing process of the drug. In this context, dynamic water vapor sorption analysis enables the precise characterization of different hydrate states and their stability as a function of temperature and relative humidity. This allows new formulations, manufacturing processes and storage conditions to be adapted to specific requirements.
Application Notes
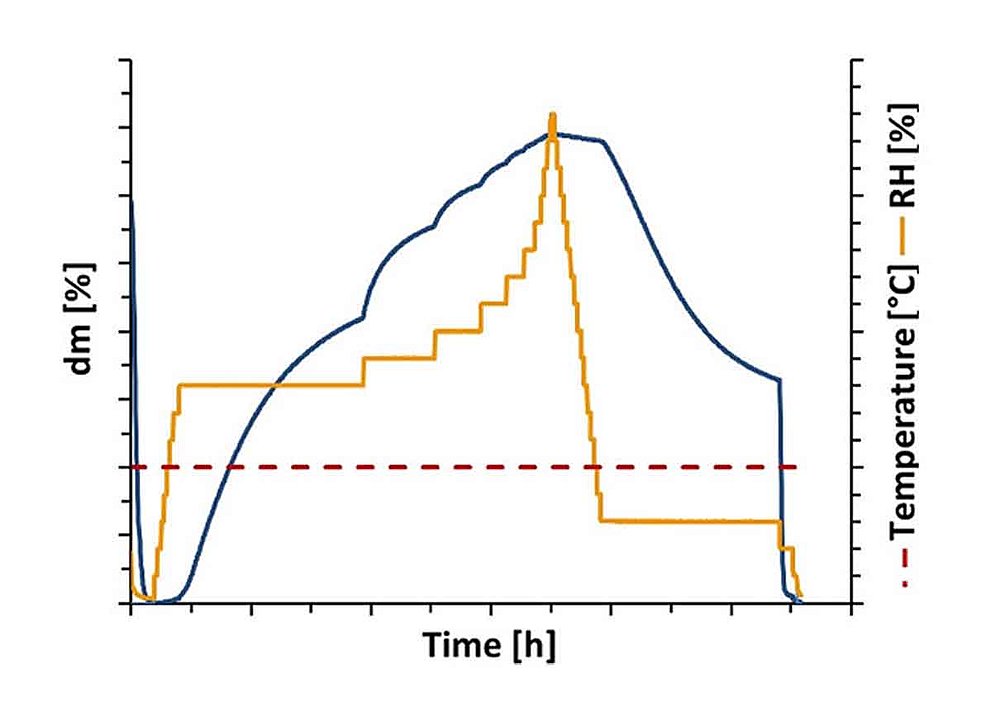
Hydrate Formation of L-lysine HCl
Crystalline active pharmaceutical ingredients (APIs) may be present in an hydrated or, after drying, also in an anhydrous state. In pharma research and quality assurance, the characterization and control of the hydrate state is highly relevant since the properties of the active ingredient are decisively influenced by the state of hydration. This includes in particular properties such as shelf life, dissolution behavior, compressibility and bioavailability of the product. By means of DVS analysis, the different hydrate states as well as their formation kinetics and stability can be characterized as a function of relative humidity and temperature. Application Note 20-06 and White Paper 20-06 demonstrate the hydrate formation of L-lysine HCl. Special focus is placed on the necessity of sufficiently long measuring times with regard to hydrate formation kinetics and the resolution of the RH steps.
Hydrate Formation of creatine
As a non-essential amino acid, creatine can be produced endogenously by the amino acids arginine, glycine and methionine. Due to its involvement in energy metabolism, it is considered to have a positive effect on muscle performance. This makes it one of the most widely used supplements in the field of sports nutrition. For creatine both an anhydrous and a hydrated state are known. Due to the influence of the hydrate form on, for example, physical properties and bioavailability, knowledge and identification of the respective state is crucial to ensure the efficacy of the substance. In the Application Note 20-09, DVS-analysis is presented as a method to determine the hydrate states of creatine and to identify critical relative humidity values for phase transition.
Literature References for Hydrate Formation Analysis with the proUmid DVS Instruments
Doris E Braun, et al. „The Eight Hydrates of Strychnine Sulfate.“ Crystal Growth & Design 20(9), 6069-6083, 2020. DOI
Matthew Allan, et al. „RH-temperature stability diagram of α- and β-anhydrous and monohydrate lactose crystalline forms.“, Food Research International 127, 108717, 2020. DOI
Matthew Allan, et al. „RH-Temperature Stability Diagram of the Dihydrate, β-Anhydrate, and α-Anhydrate Forms of Crystalline Trehalose.“ Journal of Food Science 84, 1465–1476, 2019. DOI
Doris E Braun, et al. „Stoichiometric and Nonstoichiometric Hydrates of Brucine.“ Crystal growth & design 16, 6111-6121, 2016. DOI
Martin P Feth, et al. „Challenges in the development of hydrate phases as active pharmaceutical ingredients–An example.“ European Journal of Pharmaceutical Sciences 42, 116-129, 2011. DOI
Kyriakos Kachrimanis, et al. „Effects of moisture and residual solvent on the phase stability of orthorhombic paracetamol.“ Pharmaceutical research 25, 1440-1449, 2008. DOI



