Dynamic Vapor Sorption (DVS) Analysis
The dynamic vapor sorption (DVS) method is a widely used analytical technique for studying the moisture sorption behavior of materials, applied across both industry and academia. In the pharmaceutical sector, DVS plays a key role in salt screening and polymorphism analysis. Within food science, it aids in stability and shelf-life studies while also helping to investigate moisture migration between ingredients in complex products. The technique is equally crucial in the development of advanced packaging materials, where it measures the water vapor permeability of films and foils. Additionally, DVS proves valuable for assessing the caking behavior of powdered or granular substances during storage, transport, and handling. In building physics, DVS is used to examine moisture uptake and release in insulation and construction materials, such as wood, plaster, fibers, and concrete. The electronics industry also relies on this method to assess moisture-induced failure in sensitive components. Originally developed for pharmaceutical research, dynamic vapor sorption has since found applications in an expanding range of industries, underscoring its versatility and scientific value.
DVS Systems: Specific Solutions for Your Needs
Dynamic vapor sorption is a gravimetric method to measure sorption or desorption of water by a material, subjected to different relative humidity levels at a regulated temperature. At each RH level, water uptake or water release by the specimen is recorded by frequent weighing. Detailed knowledge of the equilibrium water content over the whole relative humidity range (sorption isotherm) and the sorption kinetics is essential for handling, processing, storage and stability of a product. Depending on the sorption properties such an analysis may take up to several days. For sufficient throughput it is therefore convenient to be able to measure more than one sample at the time.
-
SPS Series
The fully automated SPS multi-sample systems offer the highest resolved sorption measurements on the market over a wide temperature range. The systems are highly versatile, enabling the simultaneous measurement of up to 23 samples supporting the use of additional sensors (e.g. Raman).
-
Vsorp Series
The Multisample Vsorp instruments are the result of our many years of experience in instrument engineering. They are designed for DVS measurements at a limited temperature range offering an excellent price to performance ratio at unrivaled versatility and accuracy.

Dynamic Vapor Sorption Method
The basic operation principle of a gravimetric sorption analyser is to measure the change in mass over time of a sample that is kept in an environment of controlled and constant temperature and relative humidity. The change in mass occurs either through sorption (absorption) of water vapor from the surrounding atmosphere or desorption (release) of water from the sample.
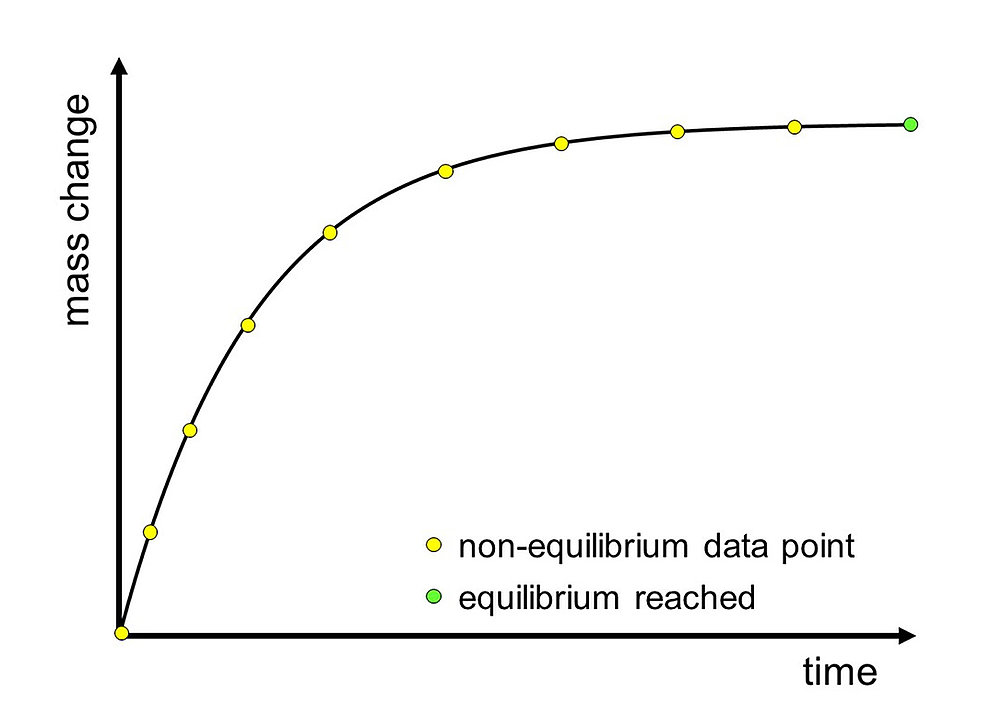
Sorption Kinetics Curve – Single Humidity Step
The absorption or release of water is continuously measured by using a microbalance. After a change in the relative humidity of the environment, e.g., an increase, the change in mass initially occurs quite rapidly. During the course of the measurement, the rate of water absorption or release decreases until the sample is finally in equilibrium with the environment. The sorption kinetics curve describes the change in mass of the sample over time at constant relative humidity and constant temperature.
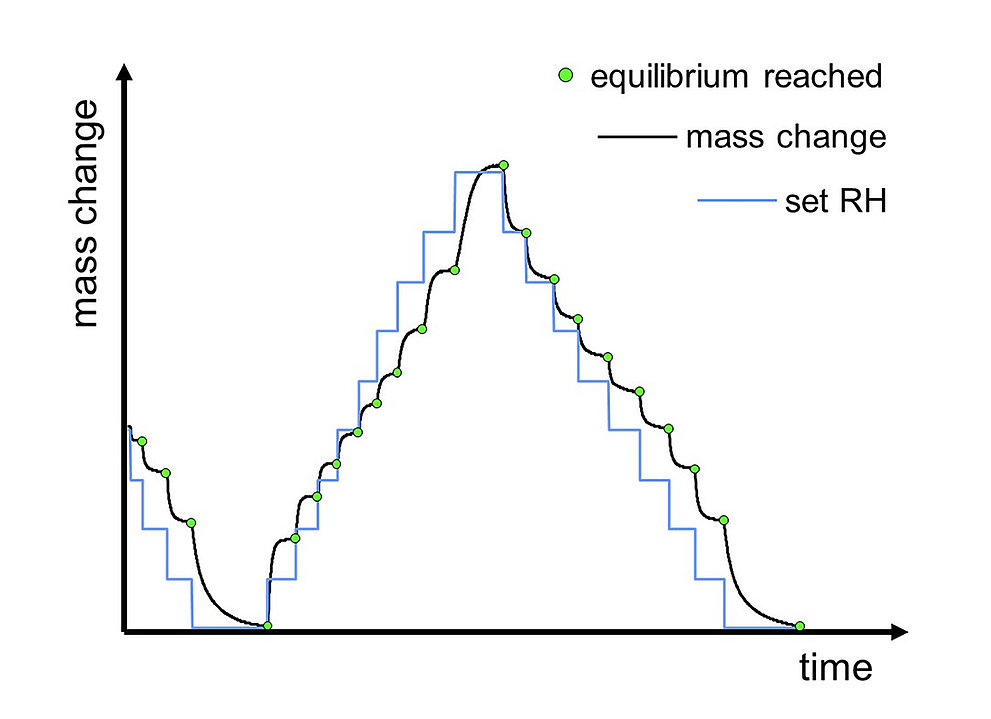
Sorption Cycle – Multiple Humidity Steps
When the sample weight is finally stable, it is at equilibrium with the surrounding atmosphere. The measurement is then continued at the next humidity step (lower or higher relative humidity). Typically, one or more complete sorption/desorption cycles (sorption from low to high RH and desorption from high to low RH) in small RH steps are done.
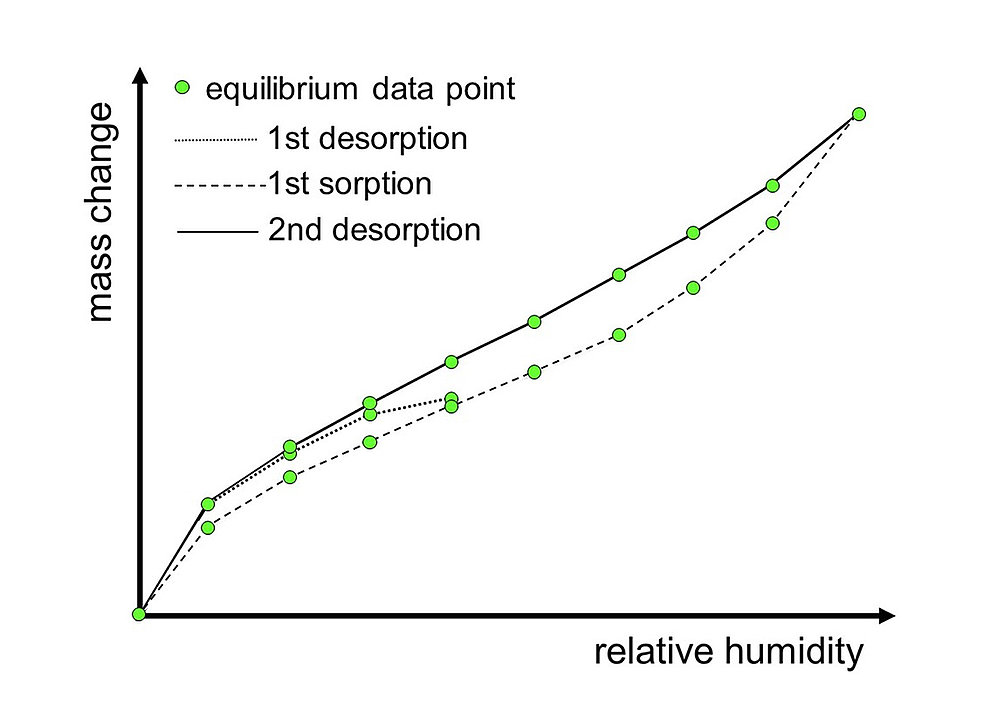
Sorption Isotherm
The individual equilibrium data points from all humidity steps of the sorption/desorption cycles are used to generate the sorption isotherm. The sorption isotherm is the relationship between the water content of a sample and the relative humidity of the ambient air at a particular temperature.



